

In summary, by controlling cytosolic calcium, NMDAR modulate mitosis and probably cell differentiation/proliferation. Phosphomimetic mutants increased entry of calcium in interphase and generated several alterations during mitosis: increased mitotic index, increased number of cells with lagging chromosomes and fragmentation of pericentriolar material. Expression of phosphomimetic mutants resulted in transient calcium influx and enhanced NMDAR inactivation independent of the cell cycle phase.
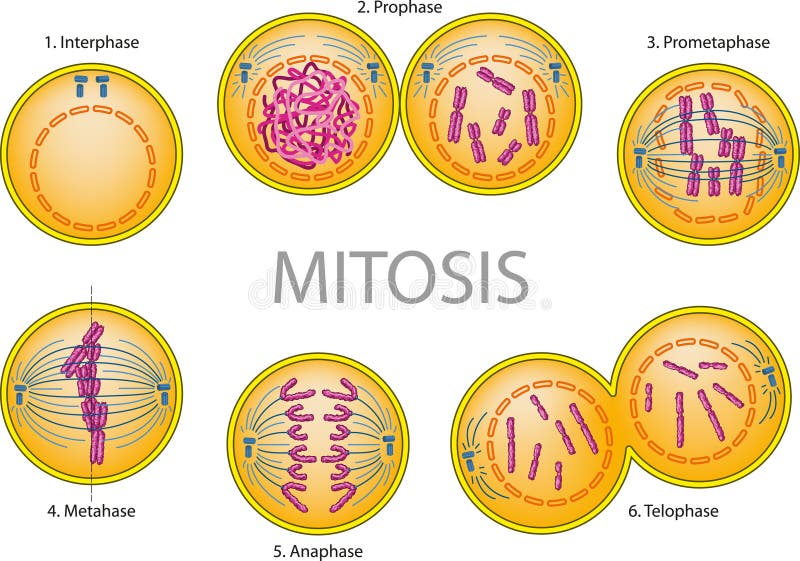
This transient calcium entry during mitosis was due to phosphorylation of the first intracellular loop of NMDAR (S584 of NR1 and S580 of NR2A) by cyclin B/CDK1. The same occurred in HEK293 cells transfected with the NR1/NR2A subunits of NMDAR. We found that neurotransmitter-evoked calcium entry via endogenous NMDAR in cortical astrocytes was transient during mitosis. However, the role of NMDAR during mitosis has not yet been explored in individual cells. NMDAR are implicated in cell proliferation under normal and pathophysiological conditions. N-methyl-D-aspartate receptors (NMDAR) are glutamate-gated calcium channels named after their artificial agonist.


 0 kommentar(er)
0 kommentar(er)
